The Opportunity
NHSX is a joint taskforce bringing together teams from the Department for Health and Social Care (DHSC) to drive digital transformation of the NHS and Social Care. NHSX has been merged with NHS Digital and now sits as part of the NHS England Transformation Directorate. With initial investments of more than £1 billion pounds a year nationally and a significant additional spend locally, NHSX was formed to give staff and citizens the technology they need.
During RedRock’s engagement, NHSX reported directly to the Secretary of State for Health and Social Care, and the Chief Executive of NHS England and NHS Improvement.
NHSX required options to address significant public health shortfalls resulting from the use of implantable medical devices (IMD). There were a number of challenges that were required to be overcome:
- Pressing timescales driven by ensuing Cumberlege report
- Compared to other areas of medicines, regulation and in-life studies not applied to the same levels
- In the instance of alerts/recalls, implanted devices cannot be traced to patients
- Diverse stakeholder groups
Our Solution & Approach
RedRock Managed Professional Service engagements are underpinned by the same core values of Client Enablement, Teams of Excellence, Impartiality and Ownership. Each engagement is directly overseen by one of our Delivery Directors to ensure this.
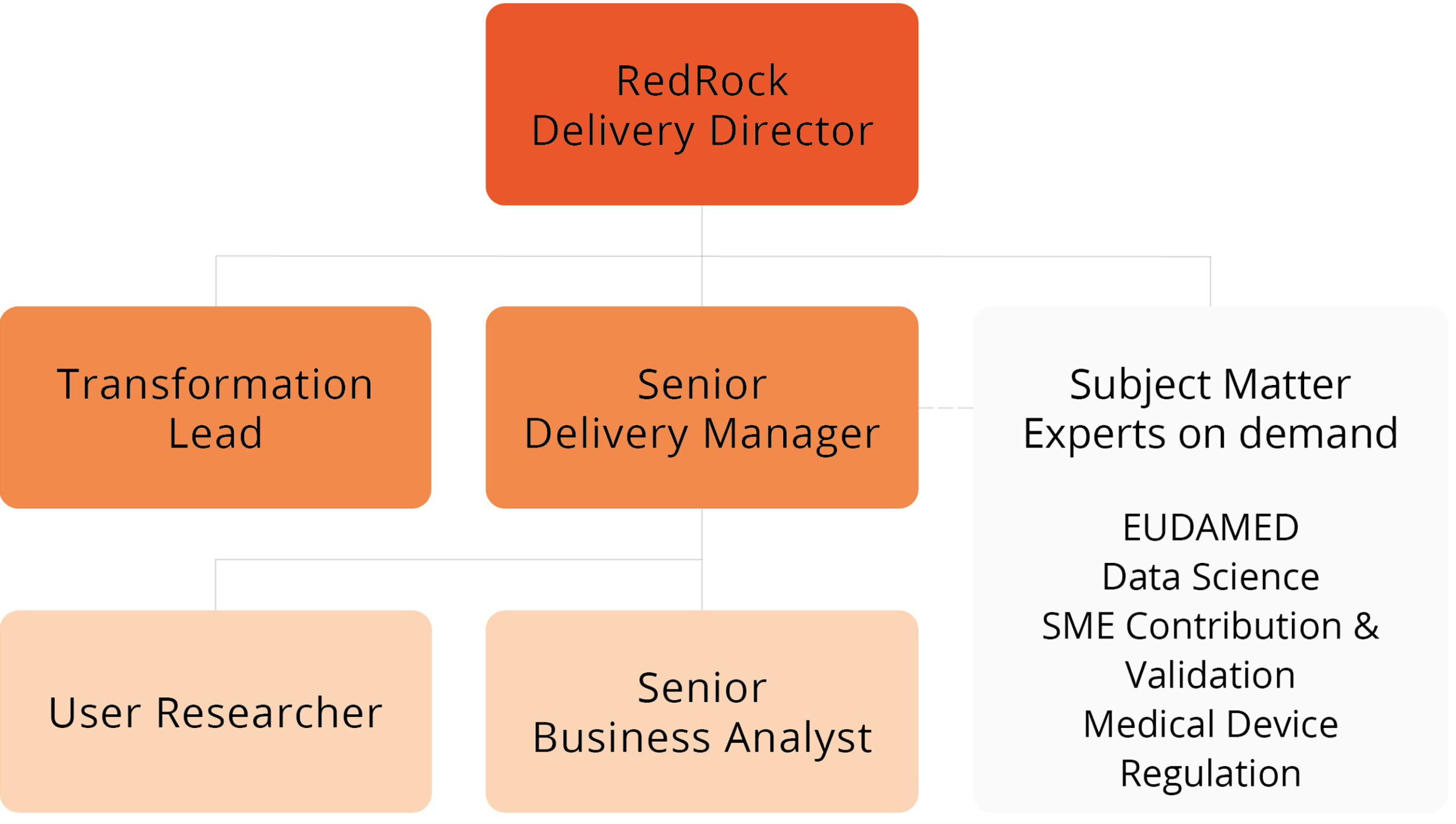
RedRock’s team consisted of a core team and we were fortunate to have access to a number of on-demand subject matter experts who were able to provide ad-hoc support and guidance to the project team.
With the limited time available, our approach centred on two practical improvement measures:
- Rapid start through success, involvement and synergies within Medical Devices at the MHRA
- Launch an IMD event creating an effective launchpad, involving C-level representatives from NHSD, NJR, IMMDS, MHRA, DHSC and Clinicians
RedRock tackled these two practical improvement measures in the following areas:
Regulation
The integration (Data & Process) of unique device identifiers (UDI) with UK MDR regulatory solution were explored. After that full UDI definition over revised range of medical device categories was carried out.
User Research
Our approach placed patients in the centre which helped us understand the end-to-end impact on people’s lives. Extensive research involving patients, clinicians, urogynaecologist, hip, vascular, orthopaedic surgeons and manufacturers was conducted including UR session observers which increased productivity. Before the project started, there was an existent need for a Patient Info Decisioning Tool which was validated after a set of interviews and research. All processes and tools that were applied followed GDS standards and practices and were documented for GDS Service Assessments.
Point of Care Traceability
A set of methods for recording devices, equipment and drugs were defined and evaluated. This included electronic data capture (barcodes & RFID technologies), Scan4Safety and Tactical scanning solution, registry levels beyond patient and device data (compliance +) and Operating Theatre procedures. Alongside error reduction, the implications of the aforementioned would also reduce the burden on clinicians
Service Design
A Six-Sigma based study was conducted. Six-Sigma is a data-driven approach that uses a statistical methodology for eliminating defects, defect reduction and profits improvement. The study defined and evaluated governance, benefits, the value model and National Joint Registry (as world leading exemplar).

The Outcome
RedRock has successfully achieved the goals set by NHSX:
- User research and personas drove our messages forwards, across a diverse group of engaged stakeholders
- People affected or involved had a voice
- Improvement measures, onward programme workstreams, structure defined
- Blended IMD Core team assembled
- Construction and launch of IMD Steering Group: driving patient safety while supporting novel procedures and innovation
- Senior Clinical Advisor nominated
- On-time, and on-budget project delivery
- The most positive meeting and outcome with the Secretary-of-State for Health: “That’s a bulls-eye”.
Ready to talk?
See how we can deliver the positive change you need. Talk to one of experts today!